T-cell receptor (TCR)-engineered T cells are novel options for adoptive cell therapy used for the treatment of several advanced forms of cancer. BioCytoceuticals specializes in performing comprehensive quality assessment on TCR-engineered cells both in vitro and in vivo, and we are more than happy to design service plan specifically catered for your goals.
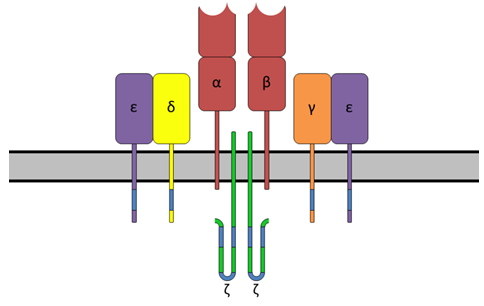
T cell specificity emerges from a myriad of processes, ranging from the biological pathways monitoring T cell signaling to the structural and physical mechanisms regulating TCRs bind peptides and MHC proteins. Binding specificity of TCR plays pivotal role in these processes. Recently, numerous pre-clinical and clinical studies have demonstrated various levels of feasibility, safety, and efficacy using TCR-engineered T cells as treatment for cancer and viral infections.
T cell activation is initiated by engagement of the T cell antigen receptor (TCR)/CD3 complex and the co-stimulatory receptor CD28. The IL-2 promoter contains DNA binding sites for NFAT, NF-kB and AP-1. Therefore, co-engagement of TCR/CD3 and CD28 leads to IL-2 synthesis, a parameter commonly used for quantification of T cell activation. To verify the quality of TCR-modified T cells prior to research practices, TCR validation assays are implemented which includes transgene expression, cytokine release, TCR/CD3ζ stability assays.
Cytotoxicity is a valuable index for TCR-modified T cells in vitro or during pre-clinical in vivo assays. Cytotoxicity of adoptive T cell that is expanded and activated in vitro is normally elevated. Therefore, a variety of methods are available to quantify the cytotoxicity of TCR-engineered T cells.
It is essential to select and construct animal models used for testing various endpoints in vivo during TCR-engineered T cell development. Customized assay protocols are designed for our clients with high reliability.
Therapeutic efficacy measurement of TCR-engineered cells during pre-clinical in vivo assays is necessary since it provides valuable guidance for potential clinical application. Additionally, although many clinical trials showed confidence inspiring results of therapeutic improvement for patients, in some cases, severe acute and chronic damage are still observed despite the engineered cells performing their proper functions.
For more details on the assays, please do not hesitate to contact us. We also provide comprehensive assays for quality control and safety evaluation of CAR cells.
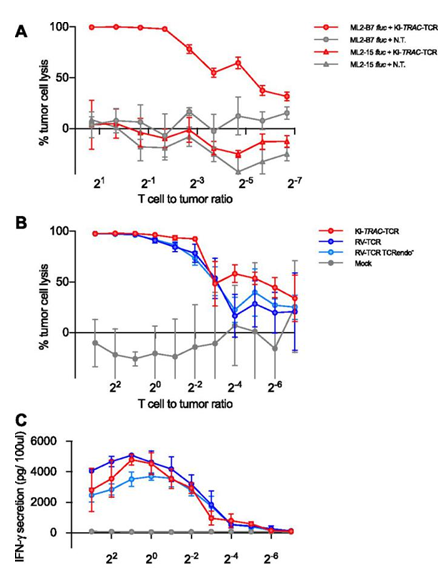 Targeted integration of a TCR into the TRAC locus redirects T-cell specificity.
Targeted integration of a TCR into the TRAC locus redirects T-cell specificity.
BioCytoceuticals leads the industry in cellular therapy with commitment to provide the most appropriate solutions to address the needs of our clients. Our expert team of scientists applies comprehensive methods and most detail-oriented scrutiny to ensure only top-tier outcome is submitted.
If you have any special enquiries regarding our TCR Cell In Vitro/In Vivo Assays service, please do not hesitate to contact us. Our highly professional technical supporting staffs will make sure your requests are faithfully received. We are looking forward to earn your business.
Inquiry