A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Cancer treatment vaccines boost the immune system's ability to recognize and destroy antigens. Some cancer vaccines are made for individual patients. These types of vaccines are produced from the person's tumor sample. Other cancer vaccines target specific cancer antigens and are given to people whose tumors have those antigens on the surface of the tumor cells. Based on Collaborative methods and deeply scientific understanding of cancer vaccine, BioCytoceuticals focuses on completing essential steps of cancer vaccine discovery, engineering, characterization and GMP production.
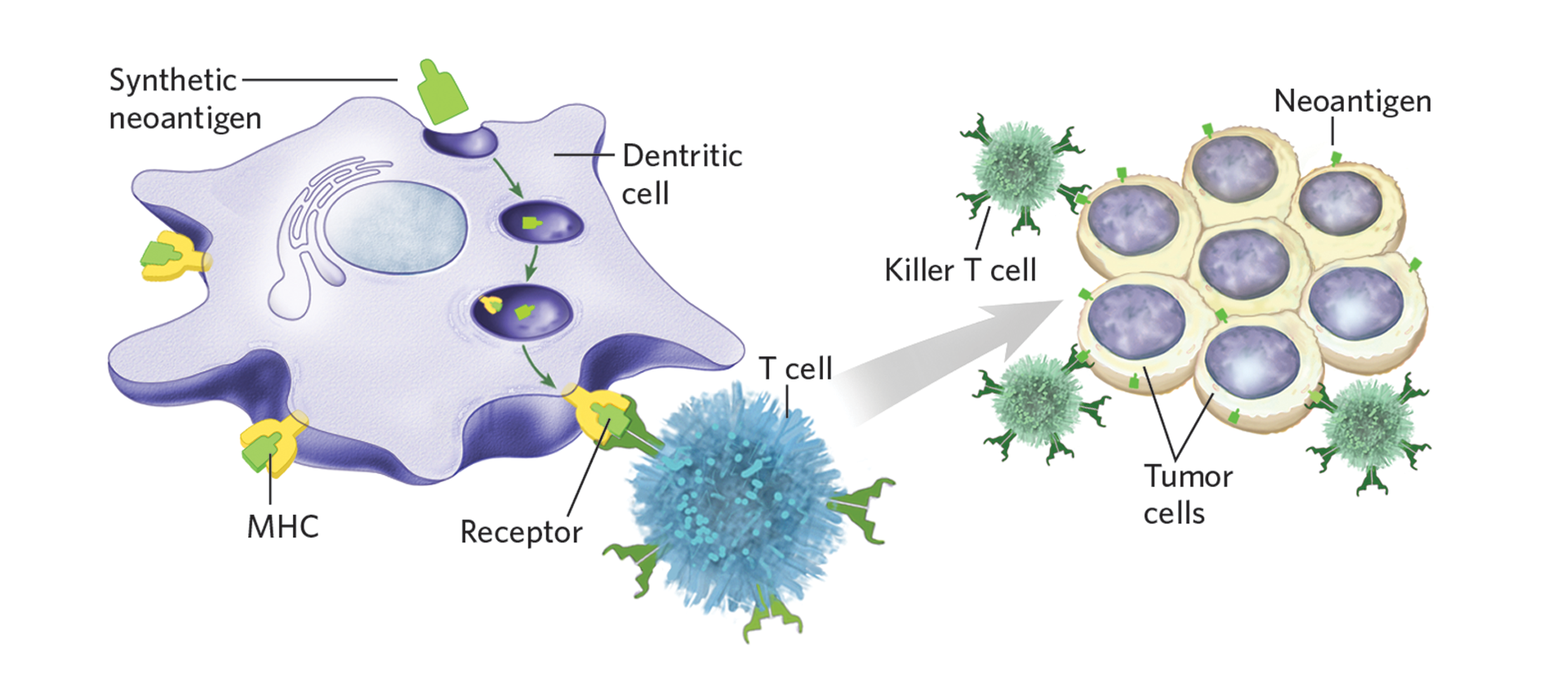
These services, including: cancer vaccine design, tumor antigen identification, adjuvant candidate selection and biomarker discovery, are available in BioCytoceuticals. Our outstanding scientists and experienced technicians are pleased to provide customized strategies and appropriate solutions for every individual service, which may help to smooth further development and successful supervision. If you have any demands in cancer vaccine discovery, or if your interests are not included in the modules, please contact us directly.
We can develop the entire processes and optimize previously individual processes to guarantee test studies meet GLP requirements. In upstream steps, selecting cell lines that best express the drug by screening a variety of production strains is significant step in entire process. Additionally, our protein analysis team will ensure product integrity during the early development process.
Cancer vaccine requires a comprehensive methodology to evaluate, and analysis for various components of the vaccine, including carbohydrate, lipopolysaccharide, lipid, protein, glycoprotein, and lipoprotein, is also critical. To help our clients obtain accurate and reliable data to report projects, we provides comprehensive characterization, including: in vitro analysis and in vivo assessment.
Cancer vaccines are developed in the same way as other biological products, but it requires high standards to ensure the purity and quality. From beginning to final steps, every step must be confirmed and the whole process must be carried out in a clean, safe, and controlled environment. In BioCytoceuticals, cancer vaccines are developed following Good Manufacturing Practices (GMP), and our cleanrooms are a part of GMP regulation for vaccine development.
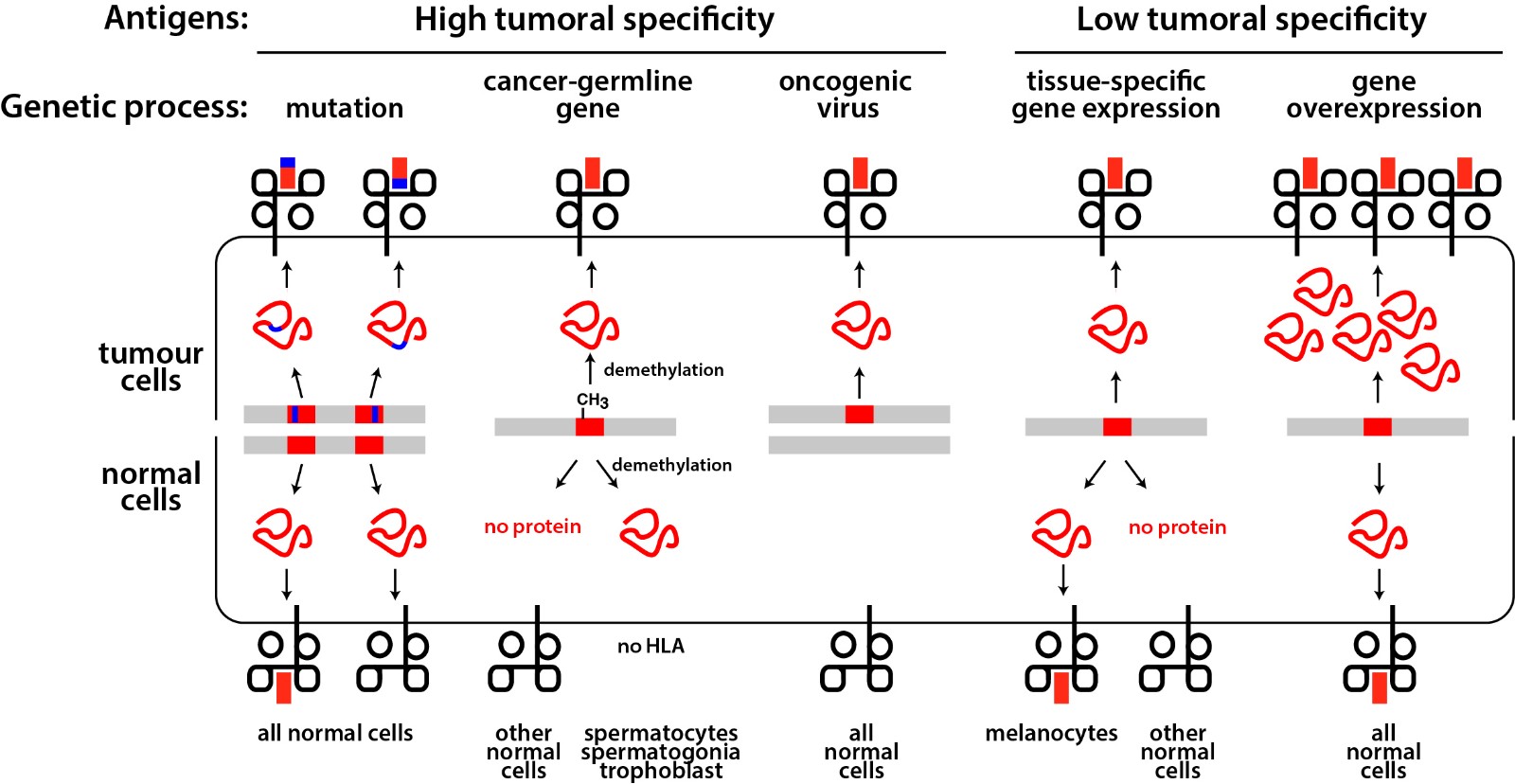
Figure 2. Tumor antigens recognized by T lymphocytes.
BioCytoceuticals leads the industry in cellular therapy with commitment to provide the most appropriate solutions to address the needs of our clients. Our expert team of scientists applies comprehensive methods and most detail-oriented scrutiny to ensure only top-tier outcome is submitted.
If you have any special enquiries regarding our Cancer Vaccine Development service, please do not hesitate to contact us. Our highly professional technical supporting staffs will make sure your requests are faithfully received. We are looking forward to earning your business.
Inquiry