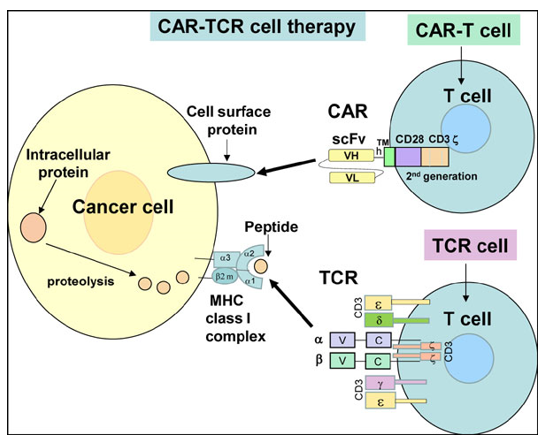
The field of adoptive cell transfer (ACT) is currently comprised of Chimeric Antigen Receptor (CAR) and T Cell Receptor (TCR)-engineered T cells and has emerged from principles of basic immunology to paradigm-shifting clinical immunotherapy. ACT of T cells engineered to express artificial receptors that target cells of choice is an exciting new approach for cancer, and holds equal promise for chronic infection and autoimmunity.
In 2017, the US Food and Drug Administration approved the first CAR-T therapies for patients with relapsed or refractory B-cell leukemia and selected B-cell lymphomas, It has high response rates in patients with refractory disease. A value analysis is required to determine whether and how to offer patients these expensive, customized drugs.
T-cell receptor (TCR)-engineered T cells are a novel option for adoptive cell therapy used for the treatment of several advanced forms of cancer. Work using TCR-engineered T cells began more than two decades ago, with numerous preclinical studies showing that such cells could mediate tumor lysis and eradication.
ScFv Generation requires highly trusted hybridoma technology, top notch antibody sequencing and recombinant antibody expression platforms, which guarantees high specificity of ScFvs. Our ScFv generation service not only includes great technology and advanced platform, but also provides immunization with a variety of antigens as well as multiple delivery options to ensure high quality performance in any and all applications.
Chimeric antigen receptor (CAR) T‐cell therapy is a new successful treatment for refractory B‐cell leukemia. After isolating T cells, the expanded T cells are purified and then transduced with a gene encoding the engineered CAR via a retroviral vector, typically Gammaretroviral vectors and Lentiviral vectors. These vectors are very safe in modern times due to a partial deletion of the U3 region.
BioCytoceuticals is an experienced and outstanding provider of Stable Cell Line Generation service. Our scientists are pleased to use extensive experience and advanced platform to offer the best service to satisfy each demand from our customers.
If you have any special need in Stable Cell Line Generation service, please feel free to contact us for this special service. We sincerely hope we can have a chance to work with you.
Inquiry