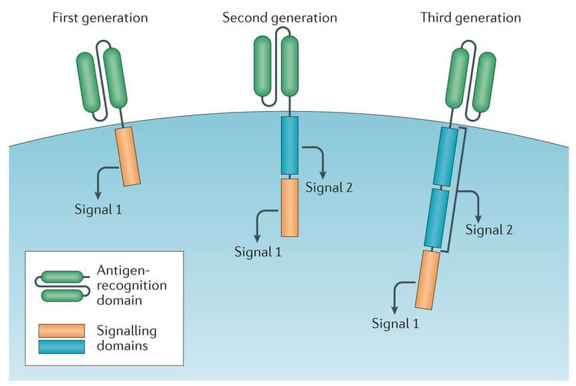
Chimeric antigen receptor T cells (also known as CAR-T cells) are T cells that have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy. The use of CAR-T cells is gaining traction as one of the most promising advances in cancer immunotherapy, which is also the first approved cell-based immunotherapy by The US Food and Drug Administration.
Several steps are required for the production of CAR-T cells. It is also very important to conduct quality control testing throughout the entire protocol.
This process requires purification autologous antigen-presenting cells (APCs) from the patients or donors, or beads coated with anti-CD3/anti-CD28 monoclonal antibodies, or anti-CD3 antibodies alone or in combination with feeder cells and growth factors, such as IL-2. IL-2 is the most commonly used agent because it induces rapid T cell growth. In order to polarize T cells to a specific phenotype, the culture conditions are further refined.
Recently, the huge success of CAR-T cells generated enthusiasm to genetically modify NK cells with CARs to sharpen their tumor-killing capacity. CAR-NK cells have several advantages over CAR-T cells.
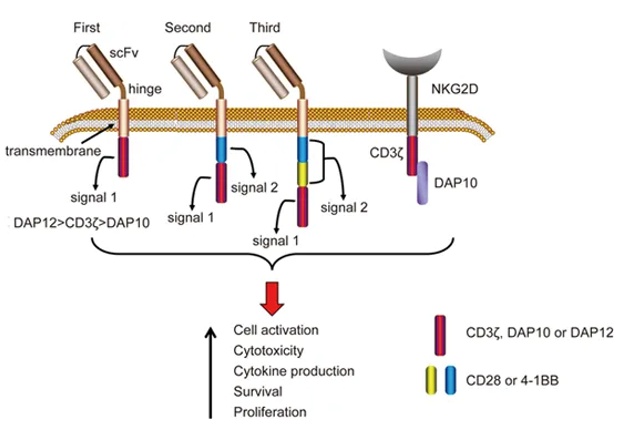
First, unlike CAR-T cells, CAR-NK cells retain an intrinsic capacity to recognize and target tumor cells through their native receptors, making the escaping of tumor cells through downregulation of the CAR target antigen less likely.
Second, CAR-NK cells do not undergo clonal expansion or immune rejection within days to weeks, and thus they do not present the same safety concerns, such as cytokine release syndrome as observed in many CAR-T clinical trials.
Lastly, NK cells do not require strict HLA matching and lack the potential to cause graft-versus-host disease, an important risk imposed by CAR-T cell immunotherapy, which makes it possible for CAR-NK cells to be an off-the-shelf allogeneic therapeutic.
BioCytoceuticals is an experienced and outstanding provider of CAR-T&-NK cell construction. Our scientists are pleased to use extensive experience and advanced platform to offer the best service to satisfy each demand from our customers.
If you have any special need in CAR-T&-NK cell construction, do not hesitate to contact us for this special service. Please let us know what you need and we will accommodate you. We are looking forward to working with you in the future.
Inquiry