Chimeric antigen receptor T cells (also known as CAR-T cells) are T cells that have been genetically engineered to produce an artificial T-cell receptor for applications in immunotherapy. The application of chimeric antigen receptor (CAR) T cells is among the most promising advances in cancer immunotherapy research while being the first of its kind to receive US FDA approval.
T-cell receptor (TCR)-engineered T cells are novel options for adoptive cell therapy used for the treatment of several advanced forms of cancer. Research using TCR-engineered T cells commenced over two decades ago. To date, there has been a array of studies demonstrating the tumor lysis mediating and eradicating potential of such cells.
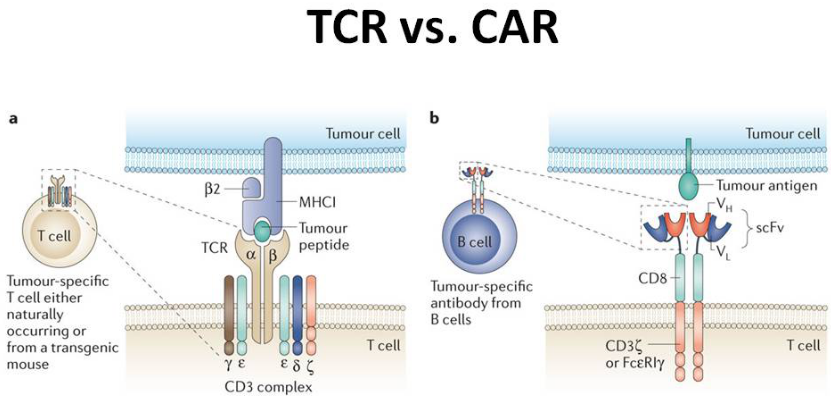
As previously stated, CAR-T cells is one of the most encouraging advances in cancer immunotherapy research. Meanwhile, research on CAR-NK and TCR-engineered T cells also showed promising potential as candidates for novel immunotherapy. These breakthroughs share a common, most crucial starting point of immune cell identification/selection.
The field of adoptive cell transfer (ACT) is currently comprised of CAR and TCR-engineered T cells and has emerged from principles of basic immunology and paradigm-shifting clinical immunotherapy. ACT using specifically engineered, artificial receptor-expressing T cells is an exciting new approach for cancer treatment which also showed potential in application to infection and autoimmunity treatments.
BioCytoceuticals provides systematic service for CAR cells in vitro/in vivo assays during CAR-T, CAR-NK, and TCR cells development.
The premise of CAR-T immunotherapy is modifying T cells to recognize cancer cells in order to more effectively target and destroy them. Scientists harvest T cells from donors, genetically alter them, then infuse the resulting CAR-T cells into patients for tumor eradication. BioCytoceuticals, depending on rich experiences and scientific understanding, employs well-established approaches for clients requesting gene package, plasmid development and T cell production.
BioCytoceuticals leads the industry in cellular therapy with commitment to provide the most appropriate solutions to address the needs of our clients. Our expert team of scientists applies comprehensive methods and most detail-oriented scrutiny to ensure only top-tier outcome is submitted..
If you have any special enquiries regarding our CAR-T GMP Production service, please do not hesitate to contact us. Our highly professional technical supporting staffs will make sure your requests are faithfully received. We are looking forward to earning your business.
Inquiry